
How powerful partnerships can unlock digital health innovation
12 min read 5 September 2024
The global digital health market is forecasted to reach $549.7 billion by 20281. This ambitious forecast is largely attributed to new startups entering the market, increased investment and the growing cost burden from the rising prevalence of chronic disease2.
The area of digital health encompasses a wide range of solutions including telemedicine, companion diagnostics, medical devices and digital therapeutics. These solutions offer huge potential for reducing pressure on healthcare systems through digital-led prevention strategies and enhancement of patient care, especially for people with at least one chronic condition as these individuals typically struggle with adherence3.
What are digital health solutions?Digital health solutions are technologies and tools that use digital platforms to improve healthcare, support patient care, and manage health more effectively. These solutions use data and software to enhance communication between patients and providers, provide personalised care and streamline healthcare processes. Examples of solutions include telemedicine, companion diagnostics, medical devices, health apps, wearable devices, AI-based diagnostics, remote monitoring and digital therapeutics.4 |
Although there are clear financial and societal benefits of bringing products to market with complementary digital health solutions, many biopharma organisations do not hold the capabilities to develop these solutions in-house. To bridge this gap and drive rapid innovation, they are increasingly turning to external partners to bring this capability.
However, despite excitement around the potential disruption from startups, partnering externally is not a guarantee of success. Research shows that while 85% of biopharma executives consider partnerships and alliances as business-critical, around half reported failure rates exceeding 60%.5
We have identified three key areas for your organisation to explore to maximise value creation and achieve long-term success in driving digital health innovation through strategic partnerships
How can a biopharma organisation prepare to partner?
Preparing for a partnership and choosing the right partner can be challenging. We therefore recommend exploring the following five considerations:
Strategic alignment
In 2023, there were over 50 partnership announcements between biopharma organisations and digital health innovators.6 High profile media attention creates a sense of urgency among biopharma organisations that do not want to be left behind. Investors are also on the look-out for investment into digital, data and AI capabilities.
When deciding to enter a partnership, it is imperative to be clear on the goals of the partnership and how it aligns with your business strategy. It is recommended to choose a partner where there are clear synergies, such as therapeutic area focus.
You should also carefully consider what partnership model would be best suited to your needs. Considerations of different models include co-development partnerships vs. co-commercialisation partnerships vs. in/out-licencing of a specific technology/IP.
The Sanofi-DarioHealth partnership is a great example of a carefully thought-out partnership designed to support patients managing diabetes, using the digital health solution, a smartphone-connected blood glucose monitor, to effortlessly complement the diabetes therapy portfolio. This successful partnership is underpinned by clear partnership objectives; 1) co-promotion, 2) product development and 3) evidence generation.7
Build vs. buy
Is it necessary to partner or can you build this capability in-house? The build vs. buy discussion should be at the forefront of your mind before you consider entering a partnership, as both options have specific considerations.
Developing a digital health solution in-house requires significant technical and regulatory expertise, including substantial resource upfront and throughout the solution lifecycle to continuously develop and maintain the solution. The interdisciplinary nature of digital health solutions requires effective collaboration across clinical science, software engineering and technology; this may clash with the legacy skillsets and capabilities that are held in-house.
Flexibility and scale of opportunities
Partnering offers access to technology experts who can design bespoke digital health solutions. Your biopharma organisation must be clear on the flexibility and scalability requirements you need from a digital health partner, to achieve mutually beneficial value in both the short and long term.
Intellectual property concerns
The rise of AI-embedded digital health solutions is generating a new wave of legal challenges concerning the ownership of intellectual property (IP), products, and patient data. Pay attention to how your partner handles issues such as IP; as your partnership progresses, alignment around these issues and how it is made accessible to patients will determine the outcomes and success of your partnership. Clearly defined contracts and boundaries can provide the guardrails and build trust between collaborating parties.
Risk appetite
When choosing a partner, you must conduct thorough due diligence to ensure the risk level is appropriate. There has been a reduced pool of potential digital therapeutics partners of late, in part due to the 2023 collapse of Silicon Valley Bank (SVB)8, and we are only just beginning to see some potential green shoots of recovery following historic low levels of digital health.9,10
When considering the type of digital health solution to invest in, it’s important to recognise the external factors that may impact success. For example, the long-term growth of digital therapeutics depends on establishing innovative reimbursement pathways, which are essential for ensuring market access and sustained financial success. Recently, to help alleviate some challenges, the Digital Therapeutics Alliance has announced an accreditation program to evaluate efficacy, privacy, security and interoperability of digital therapeutics, aiming to build trust in reimbursement decisions.11
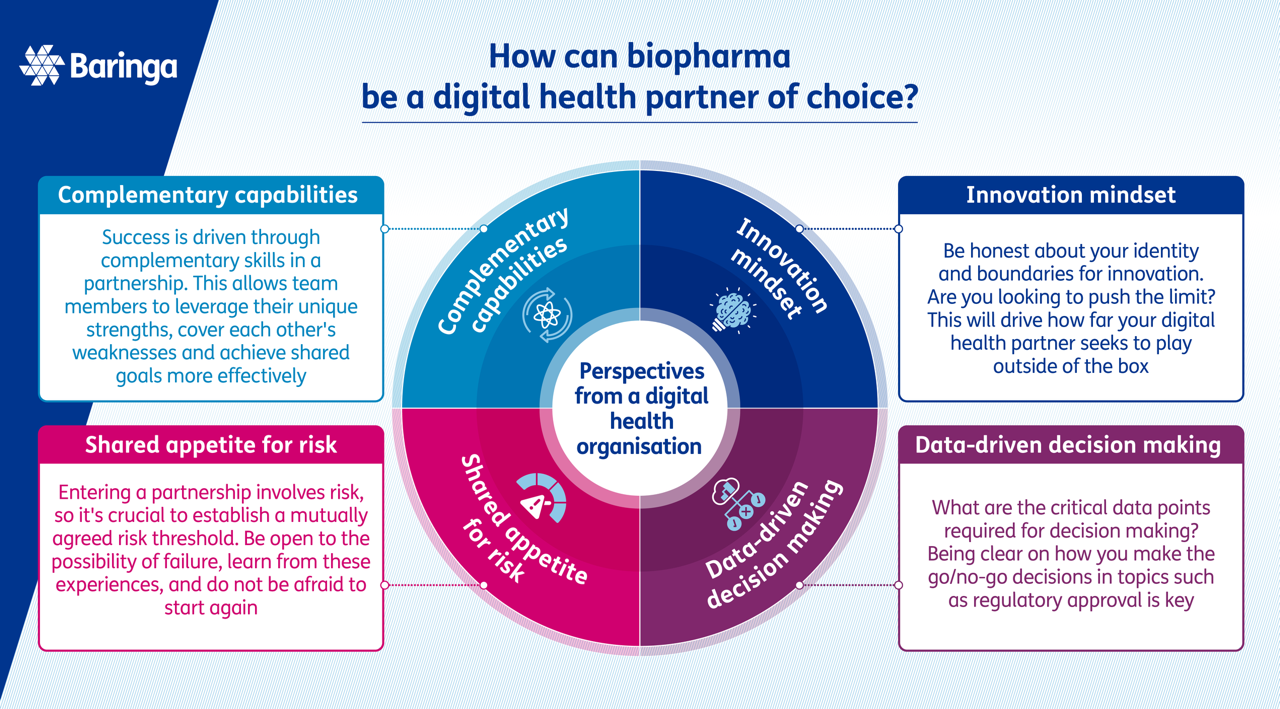
How can a biopharma be a digital health partner of choice?
Drawing in learnings from an interview with Erik Walk, Chief Medical Officer at PathAI, and our experience working across various biopharma and digital health organisations, we have identified four key qualities that biopharma organisations should consider to become a partner of choice.
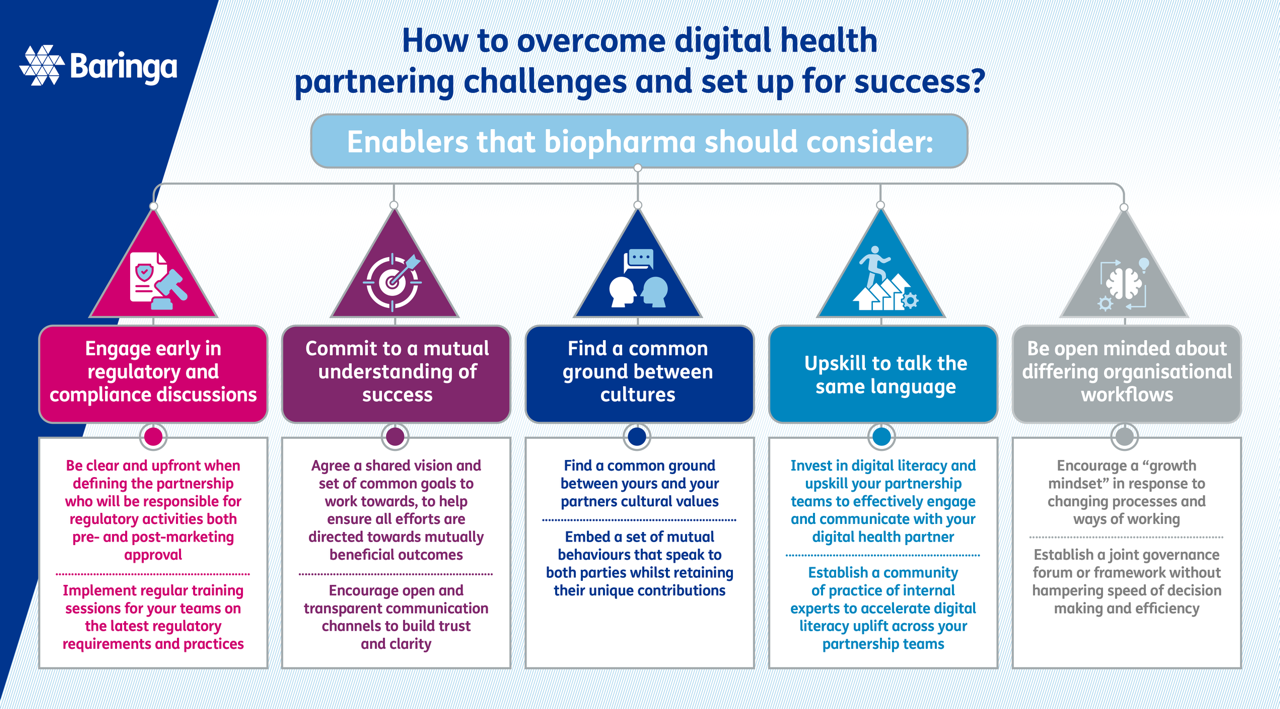
How to overcome digital health partnering challenges and set up for success?
Beneath the surface of partnerships lies a complex web of challenges that require delicate thought and careful consideration. We consider there to be five key challenges that may be experienced throughout a digital health partnership and have outlined some key enablers to help you navigate those challenges. These challenges are:
- Evolving regulatory and compliance challenges
- Differing views of success
- Opposing cultures
- Difficulties talking the same language
- Contrasting operational workflows.
Our key enablers to navigate these challenges are summarised in the infographic below.
| Challenge | How to overcome this challenge and set up for success |
| Evolving regulatory and compliance challenges |
The regulations governing digital health solutions are different to medicinal products, as they are currently regulated as medical device software in key major markets (including the UK, EU and US). The regulatory landscape for medical device software is continuously evolving. Understanding the regulatory requirements and having the resources and capability to comply will be critical to success. We recommend that whoever in the partnership is identified as the legal manufacturer of the digital health solution has an ISO 13485:2016 certified Quality Management System (QMS) in place, as having a QMS is a typical regulatory requirement. |
| Differing views of success |
Both parties in a strategic partnership should have a shared understanding of success and a common goal. This could involve a set of shared objectives and outcomes, and a joint vision statement supported by guiding principles. Have an open conversation about each other’s competing priorities, non-negotiables and risk levels to understand how best to work together. Mutual success at an individual level also plays an important role, and knowing the objectives and incentives of your alliance team can offer critical insight in setting the partnership up for success. |
| Opposing cultures |
Culture isn’t just a buzzword. When evaluating potential partners, it's crucial to assess if their culture and values align with your own. Differences will inevitably exist, but finding common ground between both partners’ cultural values is essential. From there, defining a few mutual, critical behaviours to be embedded and championed through the whole partnership top-to-bottom will enable everyone to pull in the same direction. |
| Difficulties talking the same language |
Different technical disciplines typically communicate using different terms and definitions (which are often open to misinterpretation), specialised language and often like to collaborate in different ways – it’s not a stretch to consider these challenges as akin to speaking different languages. We are seeing our clients increasingly invest in internal upskilling interventions tailored to digital, data and AI skills to bridge the growing talent and skills gap. |
| Contrasting operational workflows |
Software development timelines may be as short as two weeks whereas drug development timelines can be over a decade – this is just one example of the differing operational perspectives and associated mindsets that may be brought into a biopharma-digital health partnership. There is a very big learning curve. Recognising the complexities and differing workflows is essential as this will impact how you work together. Ensure you and your digital health partner have clear, mutually understood and contractually agreed roles and responsibilities, with a joint governance structure in place. Be open minded and embrace the change in pace for potential growth and innovation. |
Discover how Baringa can support your digital health partnerships
We work across the biopharma and life sciences sector to advise organisations on how to incorporate digital health partnerships into their R&D and commercialisation strategies, and how to maximise their value.
We typically start with an initial maturity assessment to understand the existing challenges faced with managing data, digital & technology partnerships, and recommend tailored solutions.
Get in touch with Josh Elliott to find out more about how we can help your organisation.
References
1MarketsandMarkets. "Digital Health Market." Accessed September 3, 2024. https://www.marketsandmarkets.com/Market-Reports/digital-health-market-45458752.html
2National Library of Medicine. (2024). The Burden of Chronic Disease. PubMed. https://pubmed.ncbi.nlm.nih.gov/38304166/
3Burnier, M. (2024b). The role of adherence in patients with chronic diseases. European Journal of Internal Medicine, 119, 1–5. https://doi.org/10.1016/j.ejim.2023.07.008
4World Health Organization (WHO). "WHO Guideline: Recommendations on Digital Interventions for Health System Strengthening." 2019. https://www.who.int.
5Bailey, K. (2020, November 15). The Trouble with Pharma Partnerships . . . And How to Fix Them. PharmExec. https://www.pharmexec.com/view/trouble-pharma-partnerships-and-how-fix-them
6Monk, G. (2024b, March 19). Gary Monk on LinkedIn: #digitalhealth #ai #pharma | 98 comments. https://www.linkedin.com/posts/garywmonk_digitalhealth-ai-pharma-activity-7175835722480840704-B0nX/
7MedTechDive (2023). Sanofi’s Felix Lee talks DarioHealth partnership, future of digital health. https://www.medtechdive.com/news/sanofi-felix-lee-dario-digital-health/688249/
8Mcadmin. (2023, March 15). The Impact of Silicon Valley Bank’s Collapse on Digital Health Innovation. MC AnalyTXs. https://mcatx.com/the-impact-of-silicon-valley-banks-collapse-on-digital-health-innovation/
9Chartis. (2023, April 21). The path forward for digital health as funding slows and Silicon Valley Bank fails | Chartis. Healthcare Advisory Services and Analytics | Chartis. https://www.chartis.com/insights/path-forward-digital-health-funding-slows-and-silicon-valley-bank-fails
10Market, V. O. T. (2024, July 8). H1 2024 digital health funding: Resilience leads to brilliance | Rock Health. Rock Health | We’re Powering the Future of Healthcare. Rock Health Is a Seed and Early-stage Venture Fund That Supports Startups Building the Next Generation of Technologies Transforming Healthcare. https://rockhealth.com/insights/h1-2024-digital-health-funding-resilience-leads-to-brilliance/?mc_cid=8f633c9693&mc_eid=af1543a5d1
11Nugent, H. (2024, June 6). DTA announces DTX Accreditation Program - Digital Therapeutics Alliance. Digital Therapeutics Alliance. https://dtxalliance.org/2024/06/06/dta-announces-dtx-accreditation-program/
Our Experts




Related Insights

The Power of Partnerships across the healthcare ecosystem: how can Biopharma take the lead?
The healthcare ecosystem encompasses a diverse range of organisations with a shared goal: to provide high quality and affordable healthcare. However, as this goal becomes increasingly challenging to maintain, Biopharma organisations play a key role in strategically partnering across the ecosystem to improve health outcomes.
Read more
Optimising Clinical Development ways of working in a strategically outsourced model
Transforming clinical development with a single strategic partner, reducing documentation by 36% and improving oversight and efficiency.
Read more
Securing outsourcing success through strategic relationships with third parties
Discover how strategic third-party relationships in pharma outsourcing drive innovation, efficiency, and growth, transforming transactional partnerships into powerful alliances.
Read more
Unlocking the value of Digital and AI partnerships for Biopharma: in conversation with PathAI
Explore how digital and AI strategic partnerships for biopharma companies are shaping the future of precision medicine.
Read moreRelated Client Stories

How Baringa supported Colt Technology with its CRM transformation
Join our TMT Partner Dan Nicholson, as he talks to our client Colt Technology
Read more
We talk to our client KCOM about modernising business operations
Join our TMT Director Sarah Roberts, as she talks to our client Tim Shaw, CEO at KCOM
Read more
Building a trusted data platform to enable scaling and cost savings
How do you accelerate delivery of a £multi-billion network expansion programme using advanced analytics and targeted insight?
Read more
Developing a Target State Operating Model for a technology and language services provider
Developing a comprehensive high-level target state operating model, defining the process architecture, and modelling the necessary capabilities for success.
Read moreIs digital and AI delivering what your business needs?
Digital and AI can solve your toughest challenges and elevate your business performance. But success isn’t always straightforward. Where can you unlock opportunity? And what does it take to set the foundation for lasting success?