Moving beyond a transactional to a strategic relationship with your third parties for outsourcing success
7 min read 30 January 2025
Over the past decade, outsourcing in the pharmaceutical and life sciences industry has evolved significantly, driven by factors including complexity of drug development, market pressures, and changing business strategies, with organisations harnessing in-house expertise and commoditising wider activities. As a result, the competitive landscape is rapidly evolving as we see organisations from FTSE 100 to smaller biotechs embracing the opportunity outsourcing presents at an unprecedented rate.
There is evidence of big pharma outsourcing more than 50% of their activities to third parties such as Contract Research Organisations (CROs) and Contract Development and Manufacturing Organisations (CDMOs), with while smaller to mid-sized firms outsourcing up to 100% of their R&D efforts.1 Thus indicating a substantial increased reliance on third-party vendors to deliver strategic objectives.
|
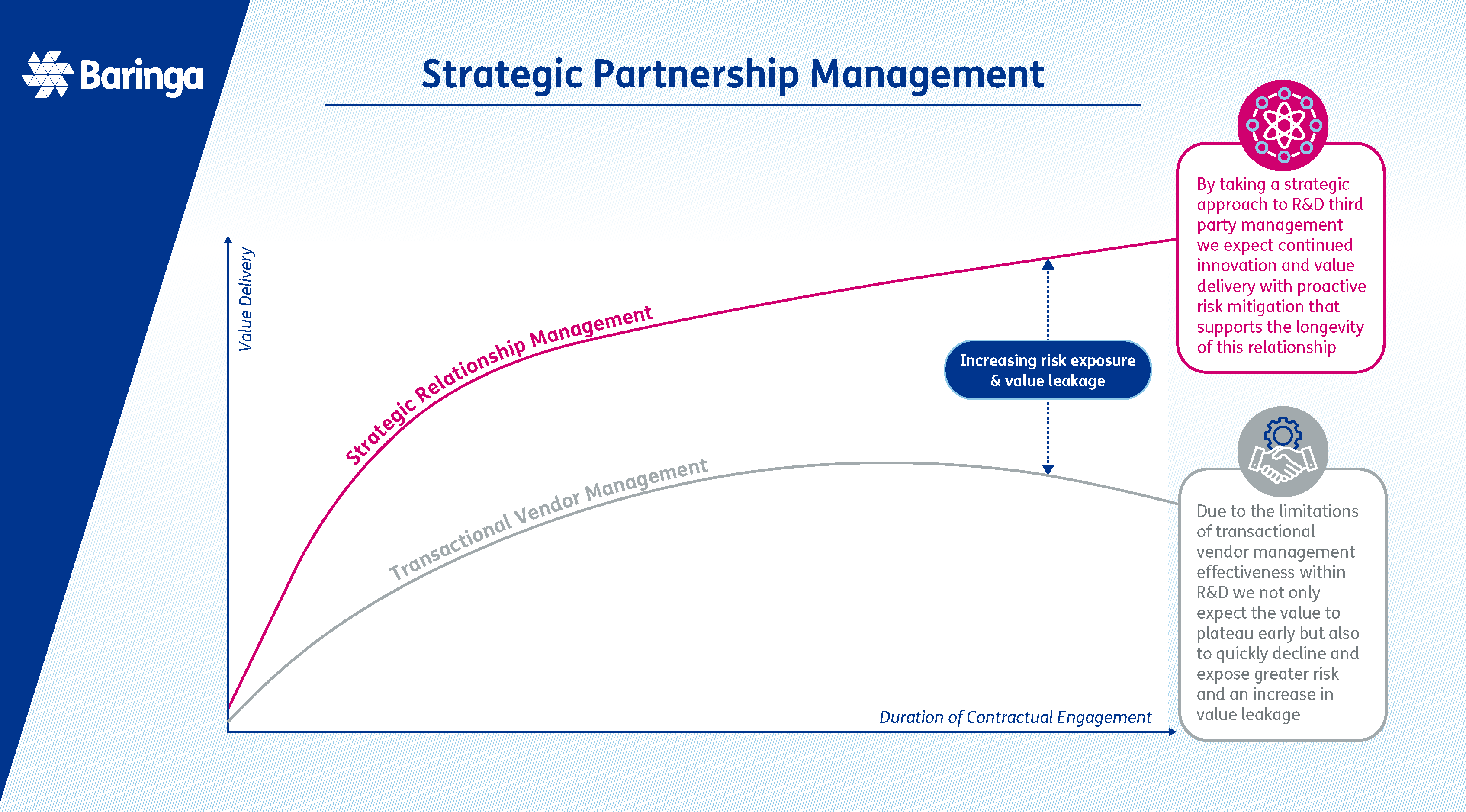
What is strategic third-party relationship management in outsourcing? Working with outsourcing partners can take many forms and happen at multiple stages of the medicine life cycle, from clinical trials to manufacturing and last mile logistics. Strategic third-party relationship management in outsourcing is an organisation’s approach to engaging and maturing dynamic partnerships with external vendors to drive innovation, efficiency, and competitive advantage, fostering a culture of mutual trust and continuous improvement. By strategically managing these relationships, organisations can unlock new opportunities for growth, streamline operations, and stay ahead in a rapidly evolving market landscape. This proactive approach transforms transactional outsourcing into a powerful catalyst for new business operating models, sustainable growth and faster delivery of the organisation’s objectives. |
In our experience, these growing partnerships with outsourced providers - while delivering a vast range of benefits - can create an additional level of complexity requiring careful and proactive management beyond what would traditionally be referred to as vendor management. The stringent regulatory environment and complex compliance requirements, for example, as well as other factors such as the scale and criticality of some of these outsources, can expose several challenges and potential risks that should be closely managed.
We highlight the key reasons why outsourcing in pharma is becoming increasingly critical, and the rationale for a renewed approach to vendor management pivoting to strategic relationship management.
What is driving the outsourcing trend in the pharma and life sciences industry?
The outsourcing trend in the pharma and life sciences industry is being driven through a combination of cost pressures, the need to access specialised expertise faster, evolving regulatory compliance requirements, and the critical need for accelerated speed to market. To explore the potential benefits of outsourcing in the pharma and life sciences industry further, we have identified the following key drivers we are seeing across the industry:
- On-demand, specialised expertise. To circumvent skill shortages or knowledge gaps internally, organisations can quickly access specialised expertise to drive outcomes faster. For example, CROs can leverage their vast internal data sets and investigational site relationships to identify the right patient populations and provide realistic recruitment predictions. This can be crucial for the success of clinical trials.
- Improved focus on core competencies. Outsourcing transactional activities enable companies to concentrate their resources and efforts on high value work, such as shaping pipeline composition, designing clinical development programs and integrated evidence plans. This strategic focus can lead to improved productivity and faster development of new products.
- Agility and scalability. By pivoting your strategy to engaging third-party services, operations can be scaled up or down based on market demands without the need to commit to hiring full-time resources. This flexibility is vital for teams to effectively respond to rapid changes in the market or the needs of a specific project or study.
- Increased efficiency. Commissioning third-party services can drive multiple efficiencies primarily through accelerated drug development and cost savings. For example, by partnering with CROs and CDMOs, pharma and life sciences organisations can access specialised expertise and advanced technologies without the need for substantial capital investments in infrastructure or personnel.
- Enhanced access to digital enablers and innovation. Third parties with specialist knowledge and expertise typically bring innovative technologies and methodologies that can improve processes, insights and outcomes, and deliver efficiency. Collaborating with these firms enables companies to remain at the forefront of industry innovation, pivoting and embedding new thinking quickly to unlock new value sources and deliver these efficiently.
Why does a transactional approach to vendor management fall short in successfully managing large, complex outsourcing partnerships?
When organisations adopt a transactional approach in their relationships with outsourcing partners, it is often due to a focus on short-term outcomes, cost-driven interactions, and siloed project engagements rather than fostering long-term, strategic relationships. This approach frequently leads to a variety of challenges in the long term as the services provided by third parties evolve and scale over time. Below, we have highlighted some of the key issues that arise from this approach:
- Strategic misalignment: Without a strategic approach to engaging outsourcing third parties, there is a risk that vendor activities may not align with the overall goals and objectives of the commissioning organisation. This may result in conflicting delivery outcomes and expectations, such as a Sponsor adopting a risk based monitoring strategy conflicting with a CRO business model of generating revenue by sending CRAs to sites. This misalignment can lead to inefficiencies, wasted resources, compliance challenges and missed opportunities for innovation and growth.
- Ensuring compliance oversight: Outsourcing activities can diminish direct control over execution, while regulatory expectations for compliance and oversight remain stringent. As third-party involvement expands with the associated fragmented contractual documentation, it becomes increasingly difficult for resource-constrained internal teams to maintain adequate oversight.
- Rigid contracts in evolving development programs: Often, contracts with CROs for studies/programs are inflexible and tied to initial assumptions that hinder the ability to adapt to changing circumstances. This can result in significant penalties or costly change orders when changes in strategy are necessary.
- Maintaining data integrity: Data serves as the backbone for pharma companies to operate, underpinning everything from clinical trials to regulatory submissions. Outsourcing partners often handle substantial amounts of sensitive information, making robust data management practices a non-negotiable necessity. However, weak and/or inconsistent controls can result in errors, missing records, or unauthorised alterations. These deficiencies pose risks beyond regulatory compliance, potentially impacting operational efficiency, financial performance, and stakeholder trust.
- Staff turnover: It is well documented2 that outsourced service providers often experience high turnover, particularly at junior levels. This can pose significant risks for pharmaceutical companies. For example, lost contextual knowledge can lead to inefficiencies and delays while disrupted relationships with external stakeholders, such as investigators and study coordinators, can further complicate projects.
- Embedded ‘Shadow’ Artificial Intelligence (AI) or Machine Learning (ML) in service delivery: Typically, pharma R&D outsourcing has focused on services requiring specialised human expertise. However, recent AI and ML advancements have made it easier than ever for service providers to integrate these technologies into their workflows,3 using them for tasks such as AI-assisted site visit report generation and automated clinical study report writing. It is therefore important that pharma companies are aware of the technology employed by their outsourcing partners and implement appropriate controls to ensure quality, regulatory compliance, and appropriate ethical standards.
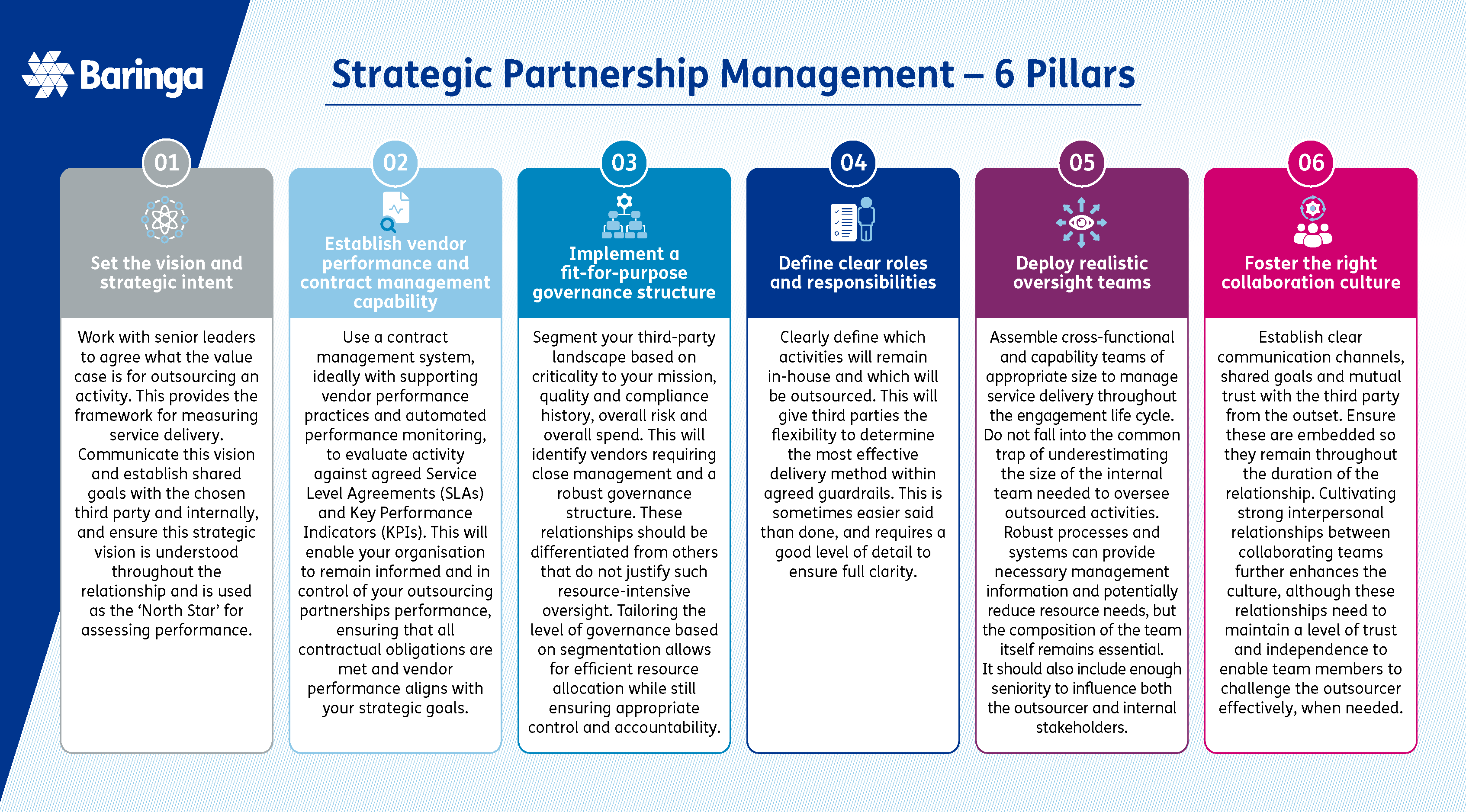
How we help our clients maximise the value of their outsourcing strategies
Through our extensive experience within the pharmaceutical and life sciences industry, and building on expertise and best practice in other sectors, we have identified effective ways of working for managing large, complex, third-party services.
In today's pharmaceutical and life sciences industry, outsourcing has become an essential component of the R&D and supply chain processes to bring innovative drugs to patients. Building an effective strategic partnership capability is therefore vital for future success. Baringa's experts can work with you to design the optimal operating model and develop the necessary capabilities to maximise the value of these third-party partnerships.
By collaborating with Baringa, you can ensure your outsourcing strategy is not only aligned with your business objectives but also enhances operational efficiency, mitigates risks, and drives innovation. Let us help you achieve sustainable growth and excellence in delivering life-changing therapies to patients. Contact us today to learn more about how we can support your journey to success.
1. https://www.statista.com/topics/11575/outsourcing-in-the-pharmaceutical-industry/#topicOverview
2. https://www.cellandgene.com/doc/the-high-cost-of-cro-turnover-and-how-you-can-avoid-it-0001
3. https://themedicinemaker.com/business-regulation/how-ai-is-changing-medical-writing
Related Insights

The Power of Partnerships across the healthcare ecosystem: how can Biopharma take the lead?
The healthcare ecosystem encompasses a diverse range of organisations with a shared goal: to provide high quality and affordable healthcare. However, as this goal becomes increasingly challenging to maintain, Biopharma organisations play a key role in strategically partnering across the ecosystem to improve health outcomes.
Read more
Optimising Clinical Development ways of working in a strategically outsourced model
Transforming clinical development with a single strategic partner, reducing documentation by 36% and improving oversight and efficiency.
Read more
Unlocking the value of Digital and AI partnerships for Biopharma: in conversation with PathAI
Explore how digital and AI strategic partnerships for biopharma companies are shaping the future of precision medicine.
Read more
How powerful partnerships can unlock digital health innovation
Strategic partnerships offer an attractive route to unlock innovation within digital health however current failure rates sit at 60%. How can you build powerful partnerships?
Read moreRelated Client Stories

We talk to our client KCOM about modernising business operations
Join our TMT Director Sarah Roberts, as she talks to our client Tim Shaw, CEO at KCOM
Read more
Building a trusted data platform to enable scaling and cost savings
How do you accelerate delivery of a £multi-billion network expansion programme using advanced analytics and targeted insight?
Read more
Developing a Target State Operating Model for a technology and language services provider
Developing a comprehensive high-level target state operating model, defining the process architecture, and modelling the necessary capabilities for success.
Read more
Streamlining organisational structure to reduce complexity, scale, and grow
A new organisational resulted in a 10% cost efficiency across the organisation, as a result of simplification, standardisation, and the elimination of role duplication.
Read moreIs digital and AI delivering what your business needs?
Digital and AI can solve your toughest challenges and elevate your business performance. But success isn’t always straightforward. Where can you unlock opportunity? And what does it take to set the foundation for lasting success?